Guide to Atmospherics: Difference between revisions
m →Atmospherics 201: Gaseous Synthesis, Machinery, Further Theory, and Optimization: Shortened this header a bit aswell. |
m →Atmospherics 301: Pipeline and Pipenet Theory; LINDA: Active Turfs & Excited Groups; Superconduction: Shortened the name of this header. |
||
| Line 583: | Line 583: | ||
*Pressures above 750 kPa do 10 DPS + 5 DPS for every extra 375 kPa above that mark, rounded off. Space suits completely block it all, but there is no other defense. | *Pressures above 750 kPa do 10 DPS + 5 DPS for every extra 375 kPa above that mark, rounded off. Space suits completely block it all, but there is no other defense. | ||
= Atmospherics 301: | = Atmospherics 301: Pipenet Theory and more = | ||
LINDA? What is LINDA? | LINDA? What is LINDA? | ||
LINDA is our atmospherics system. There are various theories on the origin of this name, but that is not why we are here. We are here to understand how gas dissipate, how pipelines and eventually pipenets are formed, and the more technical parts of atmospherics. | LINDA is our atmospherics system. There are various theories on the origin of this name, but that is not why we are here. We are here to understand how gas dissipate, how pipelines and eventually pipenets are formed, and the more technical parts of atmospherics. | ||
Revision as of 12:21, 22 May 2022
This is the Guide to Atmospherics. When properly initialized, Atmosia can keep the station aired-up through nearly any emergency. Improperly initialized, it's a waste of space at best and an outright fire hazard at worst.
We will walk through this step by step, playing with various concepts. We will discuss the more practical aspects first, and then move on to gaseous synthesis, and finally to the why and how of atmospherics. By reading this guide you will learn how to transform Atmos from a waste of space to an actually useful addition. We will go through all kinds of theory, so this may be tough, but it will also ensure you know exactly how and more importantly how Atmos works the way it does, making you ready for all kinds of situations
See also:
Atmospherics 101: Basic Theory.
You, dearest atmospheric technician, have one main purpose: Keeping the station not too hot, not too cold, pressurized, and breathable. You might be able to synthesize difficult gases, but derelict this basic duty and you are not a good atmospheric technician.
The Standard Pipes
These days, pipes no longer have a set direction they connect in. Normal pipes will automatically connect with adjacent pipes of the same color and layer. The Omni pipes (grey colored) connect with ALL colors on the same layer. This is critical to keep in mind, as careless placement of pipes can result in accidental connections between previously separate pipenets. This feature is commonly referred to as "Smart Pipes". All pipes and pipe devices can be assigned colors, and even larger atmospherics machines also can have their color adjusted.
In the Rapid Pipe Dispenser (RPD) interface, you'll notice a few green arrow buttons on the left. These can be toggled off to restrict automatic pipe connections in a direction for the pipes you place. This is incredibly handy when working in tight spaces where there's no other way to avoid having two matching colored pipes pass each other. Pressing the circle button in the center will reset the restrictions to the default auto-connect.
If you need to connect two differing colors of pipes, a color adapter can be used in place of a regular pipe. However, an Omni pipe would work fine, as well as any device set to omni like a pump or valve.
The Atmos Devices
This will be a section detailing the overall function, and some specifics, of the various pipes, pumps, and other devices. Some details will be missed, but it will provide a basis. The first instance of a device running into a unique mechanic will be explained in further length.
Canisters
These hold gases in a portable manner. However be VERY CAREFUL with them when you're dealing with hot gases or exothermic reactions (such as burnmixes, or even just freon formation), as they have pressure and temperature limits.
Canister frames may be made using 5 iron, Then completed by using 5 more iron on said frame. Canisters have a pressure limit of 500000kPa and a Temperature limit of 10000k
Canisters have a shield that when turned on prevents gasses inside the canister from damaging it allowing for extremely high pressures and temperatures as long as you have power, the higher the temperature and pressure inside, the higher the draw (it should peak around 25 kW). Power will be drawn from the nearest APC, Otherwise it will use an internal battery.
Power cells can be replaced by using a screwdriver to open the cover on the canister, then removing the battery with a crowbar.
Stationary Tanks
These are tanks that are immovable. They can be made by making a tank frame using 4 plasteel sheets and then using 20 sheets of a material of your choice on them. They have a volume of 2500L and a default pressure limit of 20000kPa. The pressure limit is affected by a material modifier, with glass giving 2000kPa and plasteel giving 30000kPa.
Useful Tips
Scrubbers, vents, injectors, valves, pumps, filters, and mixers can be safely unwrenched without spilling gas on a tile. Especially valves serve as a perfect alternative to a normal straight pipe, when wanting to be more safe with hot gas.
Most Atmospherics objects and machines can be operated with CTRL+click and ALT+click. CTRL+click will turn them on and off, ALT+click will max them out.
Panic Siphon on air alarms turns off all vents and sets scrubbers to Expanded range Siphoning. The contaminated setting will set the vents to normal atmospheric pressure, and scrubbers to Expanded range, and sucking in every gas that isn't Nitrogen or O2.
Pipes, vents, and other Atmospherics objects can be placed in walls! Most of the time, it is easier to dump gas into walls rather than trying to dump it in space. Even if the wall is destroyed or removed it will not spill the gas.
4-way Manifolds have the same volume as two pipes on top of each other, going north to south and east to west. Because of this, optimal usage of Heat Exchange pipes tends to be using a whole bunch of 4-way Manifolds.
Freezers and heaters can be placed directly on pipes, and they'll connect to it! Also, did you know that Freezers and Heaters can switch pipe layer by using a multitool on their board?
Because of the slow nature of pumps, they should be generally avoided unless looking for a specific purpose. Volume pumps are great for regulating speed consistently and pressuring pipenets because of their 9000 kPa limit. Pressure pumps are great for regulating pressure and slower gas movement, be creative!
Many, many things can be done using layer manifolds and different piping layers. No two ways about it, you'll have to experiment!
The Atmos Tools
Main page: Atmospherics items
Rapid Pipe Dispenser
This is your Rapid Pipe Dispenser. There are many like it, but this one is yours. The main tool of your trade, the RPD is what you use to generate new atmospheric devices. Use it in modifying the station's layout, in making new production compounds, in adding scrubbers and vents to various parts of the station, and much, much, more. Use it and use it well. Keep in mind that this device sparks when changing selections, and it sparks a lot. Sparks can create fires if there are flammable gases around.
Analyzer / BreathDeep Catridge
Where the RPD is your sword, the Analyzer  is your eye. Use this in identifying various gases in various pipelines (more on pipelines later), in analyzing the air around you, in identifying problems, harmful gases, and other various atmospheric related occurances.
This is slightly less related to the job, but the Analyzer also has a barometric function; giving you information on incoming storms when Alt-Clicked on planetary environments.
is your eye. Use this in identifying various gases in various pipelines (more on pipelines later), in analyzing the air around you, in identifying problems, harmful gases, and other various atmospheric related occurances.
This is slightly less related to the job, but the Analyzer also has a barometric function; giving you information on incoming storms when Alt-Clicked on planetary environments.
ATMOS Resin
The Backpack Firefighter Tank![]() can switch modes to launch transparent ATMOS resin instead of extinguisher. This resin has the following effects:
can switch modes to launch transparent ATMOS resin instead of extinguisher. This resin has the following effects:
- Repairs hull breaches similarly to Metal Foam.
- Cleans the air from toxins.
- Normalises air temperature to room temperature (20°C or 293.15K).
- Seals open vents (as if they were welded shut).
- Removes slipperiness from floors (from water etc).
- The foam itself is not slippery.
To use the Backpack Firefighter Tank, equip it on your backpack slot and click the new hud icon to take out the nozzle![]() . You can then cycle modes between extinguisher, resin launcher and single tile resin launcher (foamer) by activating the nozzle in your hand. It spends water when used. Examine the nozzle to see water remaining. This anti-breach and firefighting tool can be ordered from cargo or found in atmospheric lockers.
. You can then cycle modes between extinguisher, resin launcher and single tile resin launcher (foamer) by activating the nozzle in your hand. It spends water when used. Examine the nozzle to see water remaining. This anti-breach and firefighting tool can be ordered from cargo or found in atmospheric lockers.
ATMOS Holofan
The holofan  is a wonderful tool that can project up to 6 holographic barriers which block gas movement. You can use these holofans to isolate breaches, prevent a spill from getting worse, or even to do basic retooling of the atmospheric layout (such as using a holofan on a pressurized plasma pipe you are about to unwrench). Keep in mind however, that holofans does not block the superconduction (explained later) of hot gases and is less reliable in very high temperature environments, especially when fire is involved.
is a wonderful tool that can project up to 6 holographic barriers which block gas movement. You can use these holofans to isolate breaches, prevent a spill from getting worse, or even to do basic retooling of the atmospheric layout (such as using a holofan on a pressurized plasma pipe you are about to unwrench). Keep in mind however, that holofans does not block the superconduction (explained later) of hot gases and is less reliable in very high temperature environments, especially when fire is involved.
Atmospherics and The Station
Over the course of the shift, various parts of the station might regrettably explode and vent out your precious, precious air. This is where you come in. Grab your nearest metal or Rapid Construction Device  and rush off to seal the breach. Grab adequate pressure and temperature protection if you have not. If your distribution loop is ready, refilling should be absolutely easy. Rush to the air alarm in the corresponding area and fill the air by interfacing with the Air Alarms File:AirAlarm.png. You might expedite the process by setting the operating mode to "Refill" or even to turn off external pressure checks by manually setting each vents under "Vent Controls" to Internal and 0 kPa. Alternatively, hauling some canisters of air
and rush off to seal the breach. Grab adequate pressure and temperature protection if you have not. If your distribution loop is ready, refilling should be absolutely easy. Rush to the air alarm in the corresponding area and fill the air by interfacing with the Air Alarms File:AirAlarm.png. You might expedite the process by setting the operating mode to "Refill" or even to turn off external pressure checks by manually setting each vents under "Vent Controls" to Internal and 0 kPa. Alternatively, hauling some canisters of air  , a portable pump filled with air
, a portable pump filled with air  , or even deploying a few Proto-Nitrate crystals
, or even deploying a few Proto-Nitrate crystals  if you've made some can all help refill the air even faster.
if you've made some can all help refill the air even faster.
Over the course of the shift, the environment might become cold or hot too. Common sources of coldness include space and icemoon wind rushing in, check for opened airlocks! Common sources of heat are usually gas combustion 

 and Pyroclastic Anomalies. To combat the temperature problems, there are many things that you could do:
and Pyroclastic Anomalies. To combat the temperature problems, there are many things that you could do:
- You could utilize Space Heaters

- You could cycle the air File:AirAlarm.png; be it from the "Cycle" Operating Mode or from manually Panic Siphoning and setting the air to Refill.
- You could even build a thermomachine
 and haul a portable pump
and haul a portable pump  to a location and keep cycling the air locally. This will heat up or cool down the air very quickly.
to a location and keep cycling the air locally. This will heat up or cool down the air very quickly. - Your trusty ATMOS Resin
 will also set air to 20 degrees Celcius and might be of great use here, but keep in mind that it seals scrubbers and vents off.
will also set air to 20 degrees Celcius and might be of great use here, but keep in mind that it seals scrubbers and vents off. - You also have the holofan
 to prevent the cold from spreading further.
to prevent the cold from spreading further. - If you've made them in the Crystallizer, Healium crystals
 are quick and effective at correcting temperature issues. Just activate and throw em in!
are quick and effective at correcting temperature issues. Just activate and throw em in!
You have great tools at your disposal, but also a great adversary to face. Good luck in your job as the Custodian of The Air!
Atmospherics 201: Further theory and Gases
The Gases and Their Functions
Let's start with some theory about the gases. Below are the different gases that can be found in-game.
Quick note: The endothermic and exothermic descriptions in these gaseous reactions are measured with respect to enthalpy. Heat capacity can change, and this means that there might be cases where you have an exothermic reaction but the temperature is actually falling. Experiment!
Gaseous Export: Gas canisters can be exported through cargo in exchange for money. They are however, subject to elasticity and will give diminishing returns for each mole exported. Gases will roughly fall to half their credits per mole value at around the 2100 moles mark, quartering at 4201 moles, and becoming one tenth of their original base export price at 6978 moles. This diminishing returns are tracked individually per canister.
| Gas | Description | Reaction | Export price |
|---|---|---|---|
 O2 (Oxygen) |
Our first base gas is Oxygen. All humans, pets, lizard-people and you name it need more than 16 kPa of oxygen in the air or internals to breathe. Any less and the creature starts to suffocate. Oxygen is an invisible gas. To detect it, use your PDA or a wall mounted Air Alarm. Oxygen canisters are marked in blue. Emergency Oxygen Tanks, filled with about 300 kPa, spawn in your emergency Internals Box. Larger Oxygen Tanks are in Emergency Lockers all across the station, which start with about 600 kPa. |
N/A | 0.2 credits per mol. |
 N2 (Nitrogen) |
Our second base gas is Nitrogen. Not particularly more heat absorbant than any other gas. However, it cannot burn at all, which may slow down fires simply by taking up space. It can reduce the heat penalty on the SM, which will keep temperatures down. | N/A | 0.1 credits per mol. |
 Air |
A 1:4 gasmix of O2 and N2 (20% O2, 80% N2). The station is filled with this.
Air in SS13 can be seen, strangely enough, as a 'watered down'-O2, with N2 being the water. Optimal atmospheric pressure for humans is 101.3 kPa. Due to the minimum of 16 kPa of O2, the pressure of 101.3 kPa cannot be changed too much without the situation becoming excessively lethal. Under 16 % oxygen? You start dying. Under 90 kPa due to fire from a while ago? You start dying. Be mindful of this. |
N/A | N/A |
 CO2 (Carbon dioxide) |
The third gas available for atmosians from the start of a shift: Carbon Dioxide. What the fuck is Carbon Dioxide!? It's an invisible, heavy gas. It chokes people effectively and quickly, and if you can be bothered to set the alarms up, will result in a invisible room that kills those in it. Takes some setup and can be very, very annoying. Causes people to gasp at low levels. It is also often used to beef up the power generation of the Supermatter Crystal. | N/A | 0.2 credits per mol. |
 Plasma |
Our fourth and the most infamous of the base gases: Plasma, a.k.a. Toxins. Plasma is purple, toxic, and flammable. When ignited in an oxygenated room it will produce fires. Plasmafires use oxygen and plasma to produce heat and waste gas. Energy released from plasmafires depends on the burn rate for plasma. The plasma burn rate itself depends on the composition of the air and the temperature of the burn. Optimal composition for maximum burn rate is 10x more O2 than Plasma, with the air temperature exceeding the upper limit of 1643.15 Kelvins. Oxygen is burned at 0.4x the rate of plasma at temperatures above the upper limit. More oxygen (up to 1.4x the plasma burn rate) will be consumed for lower air temperatures. |
The aforementioned waste gas of plasmafires are either solely tritium on oxygenated plasmafires (more detail on the tritium section below) or water vapor and CO2 on a 3 CO2 : 1 H2O ratio on non-oxygenated plasmafires. | 1.5 credits per mol. |
 N2O (Nitrous oxide) |
The final base gas available in the atmos tanks: Nitrous Oxide, a.k.a. Sleeping Agent. A white-flecked gas.
Makes you laugh at low doses and at higher ones puts you to sleep. If using this as a sleep gas mix do not forget to mix in at least 16 kPa of O2, or you will suffocate someone. |
This decomposes into Nitrogen and Oxygen at temperatures at or over 1400K, creating Nitrogen equal to the amount of N2O used, and half that amount in Oxygen. | 1.5 credits per mol. |
 Tritium |
Radioactive, flammable gas that is used in plenty of chemical reactions. Created by heating loadsa O2 with Plasma. Emits radiation when combusted in the air, as well as pipes and canisters. Might not want to put this into any engine unless you plan to set it on fire.
Important to remember is that tritium will likely be very hot when exiting the chamber, opening possibilites of cracked canisters and eventually toasted incinerators. Prepare accordingly! It is also worthy to note that tritium when allowed to react with oxygen will burn up into water vapor. Due to the chamber having a lot of oxygen, it is often a good idea to add a second scrubber to prevent too much tritium from being lost. Keep this in mind when attempting to get sizable amounts of it. Tritium burns in a unique fashion. There are two ways tritium can burn, a very energetic high reaction rate burn and a slower one. The former is a key component to bomb making due to the insane pressure hop on the burn tick. The high burn rate occurs when there are more oxygen than tritium in a given mix AND if the total energy of the turf is above 2 million joules. If these requirements aren't met the burn reaction will revert to the slower one which wont be as theatrical as the first one. |
Tritium is created in fires that are super saturated, i.e. fires where there are 96 more units of oxygen than plasma. One popular ratio used by many Atmosians is 97 O2 : 3 Plasma, this wont hit the super saturation threshold from the get go, but given time the oxygen input will overflow the oxygen burn rate, resulting in a net positive oxygen gain in the chamber and eventually hitting the threshold. This oxygen accumulation continues over time, and therefore it is a good idea to lower the oxygen ratio in the burn mix over time. Another popular mix for chambers that have burned for quite some time is 85 O2 : 15 Plasma. | 2.5 credits per mol. |
 H2O Water Vapor |
Pure H2O. Keep away from the Clown - this slips people and even freezes tiles when released at low temperatures. The Janitor starts with a tank in his closet; | created as a waste product on tritium fires and unsaturated plasma fires. | 0.5 credits per mol. |
 H2 (Hydrogen) |
Hydrogen is a flammable gas which when ignited burns similarly to tritium. It is also an integral part of fusion reactions. Hydrogen can be solidified in the Crystallizer with BZ as catalyst at high heat and pressure (between 50,000K and 150,000K) to produce metal hydrogen |
Hydrogen is made by electrolizing Water Vapor with an electrolyzer machine. | 1 credit per mol. |
 BZ |
BZ gas is a potent hallucinogenic that also puts slimes into stasis and degenerates changeling chemicals. As a side effect, affected people will take low brain damage. BZ sees frequent use as an ingredient/catalyst in many gas reactions.
If mixed in a tank with oxygen, it can be used for internals, to encourage spiritual development. Breathing it also produces BZ Metabolites. |
BZ is formed in an exothermic reaction when at least ten moles of each N2O and Plasma are combined at low pressures. The optimal pressure for this is 0.1 atmosphere, or about 10 kPa. Efficiency might be higher if you get it even lower somehow, though. Plasma is consumed at 2x the rate of N2O. A good mix to use is 66 Plasma : 33 N2O | 1.5 credits per mol. |
 Pluoxium |
A non-reactive Oxygen substitute that delivers eight times as much O2 to the bloodstream, with as little 3 kPa minimum pressure required for internals! | Pluoxium may be created by exposing O2, CO2 and Tritium together in an exothermic reaction between 50 K and 273 K. This reaction creates a minimal amount of H2 (1% of Pluoxium created) as a byproduct. The consumption ratio for this reaction is 100 O2 : 50 CO2 : 1 Tritium. | 2.5 credits per mol. |
 Miasma |
Miasma smells bad and can cause diseases to spontaneously appear. The higher concentration of miasma in the air, the higher level symptoms can appear. | Miasma (bad air) is created from bloomed Corpse Flowers. Sterilized into oxygen in a slightly exothermic reaction at 170 degrees Celcius. Presence of water vapor in quantities higher than 0.1 moles prevents this from happening. This reaction has the lowest priority out of all reactions in the game. It is otherwise absolutely inert in terms of atmos reactions. |
1 credit per mol |
 Nitrium |
Nitrium (a combination of the old gasses Nitryl and Stimulum) is a gaseous stimulant that when inhaled can enhance speed and endurance. At low concentrations Nitrium will increase your top running speed while healthy and unimpaired. At slightly higher concentrations breathing Nitrium will form Nitrosyl plasmide in the bloodstream, providing immunity to stuns and sleeps. This is in addition to the speed boost. Damage slowdown from stamina damage (stun batons!) will still slow you even with the stun immunity. At high concentrations breathing it will damage a person's lungs.
Nitrium decomposes exothermically when in contact with Oxygen under 343.15 K, splitting into a 1:1 mix of Nitrogen and Oxygen. Meaning you will have to experiment to find a way to breathe Nitrium and not suffocate while doing so if you wish for ultimate power. Breathing Nitrium in high concentrations will quickly cause lung failure, make sure that Nitrium makes up a minority of your tank. |
Nitrium is made by combining a minimum of 20 moles Tritium, 10 moles Nitrogen and 5 moles BZ in a (slightly) endothermic reaction above 1500K. The consumption ratio for the reaction is 20 N2 : 20 Trit : 1 BZ. Higher heat improves the rate of reaction. Also formed in high quantities by fusion. | 6 credits per mol. |
 Freon |
On temperature lower than 0°C (273.15 K) Freon will create an endothermic reaction with O2, meaning it will absorb heat from the atmosphere, down to a minimum close to 50K. Adding Proto-Nitrate will catalyse the reaction so that it may begin at temperatures up to 310 kelvin, which is above room temperature. This reaction produces CO2 and if the temperature is between 120-160K the reaction has a small chance to also produce solid sheets of hot ice Breathing Freon causes burn damage. Hot ice is a solid byproduct of the cooled Freon+O2 reaction at 120-160K. Can be sold to cargo at a high price. It holds a great amount of power inside. Can be ground to produce 25 units of Hot Ice Slush. If hit with a welder or burned the hot ice will melt, releasing the power stored inside. This releases large amounts of hot plasma into the air. (Moles of plasma released = 150 x number of sheets) and (Heat released = 20 x number of sheets + 300K). |
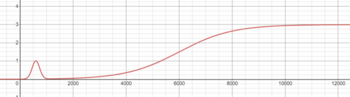
Freon is made by combining a minimum of 0.6 mol of Plasma, 0.3 mol of CO2 and 0.1 BZ, with reaction speed depending on temperature, as depicted in the graph below. The reaction is endothermic. The consumption ratio for the reaction is 6 Plasma : 3 CO2 : 1 BZ, forming 10 moles of Freon. Unless you're able to push the reaction into high temperatures, it is best to try and maintain a temperature of 800K. The energy consumed by the reaction also scales up as temperature increases, so it may be harder to maintain a high temperature than one might expect.
|
5 credits per mol. |
 Hyper-Noblium |
Extremely inert, Hyper-Noblium stops other gases from reacting. (Specifically, it stops reactions when >5 moles and temp > 20 K) | Hyper-Noblium Can be created when Nitrogen is combined with Tritium at extremely low temperatures (below 15 K). Reaction produces significant energy (exothermic) and BZ works to reduce the energy released, expect to have your temperature spike if you don't use BZ, the energy released is potent enough to be used for explosives! 10 mol of Nitrogen is used per mol of Hyper-noblium synthesised, and you also need at least this much to have the reaction occur. 5 mol of Tritium is the minimum required to have the reaction occur, and is the amount used when no BZ is present. However, the amount of Tritium used scales with the ratio of Tritium to BZ, all the way down to 0.005 mol used in a ratio of 1:1000 Tritium:BZ. In short: keep your BZ high and your Tritium low if you want to make a lot of this stuff! | 2.5 credits per mol. |
 Proto-Nitrate |
Proto-Nitrate is a highly reactive gas, but non-toxic when inhaled.
|
Proto-Nitrate is created in an exothermic reaction when Pluoxium is exposed to H2 at temperatures between 5000-10000 K. Hydrogen is consumed at around 10x the rate of Pluoxium. | 2.5 credits per mol. |
 Halon |
Halon acts as a fire suppressant by removing oxygen in the air (while producing CO2) in an endothermic reaction if the air temperature is above 100 C or 373.15 K. The oxygen suppresion rate is 20 O2 : 1 Halon. | It is created in a slightly exothermic reaction between CO2 and N2O in turfs with an active electrolyzer on them, below 230K. 2 moles of CO2 are used and 1 mol of N2O is used. | 4 credits per mol. |
 Healium |
Healium (not to be confused with actual Helium) is a red gas which acts as a stronger sleeping agent than N2O, while healing burns, bruises, suffocation and toxin damage. | It is created by exposing Freon to BZ in an exothermic reaction at temperatures between 25-300 Kelvin (keep it chill). Freon is consumed at around 11x the rate of BZ; a little bit of BZ will very quickly transform all of your Freon into Healium if you're not careful. | 5.5 credits per mol. |
 Zauker |
Zauker is an incredibly deadly gas if inhaled. | Zauker is made by mixing Hyper-Noblium and Nitrium in an endothermic reaction at temperatures between 50000-75000 K. Nitrium is consumed at around 50x the rate of Hyper-Noblium. It is worthy to note that Hyper-Noblium stops reactions when it is present in quantities above 5 moles, prepare accordingly! Zauker also decomposes exothermically into a 30/70 O2/N2 mix when exposed to Nitrogen. |
7 credits per mol. |
 Helium |
Helium is an invisible, inert gas. It has minor use within the Crystallizer to make a Crystal Cell, but otherwise is functionally useless. Sell it to cargo! | Helium is produced as a common byproduct of fusion in the Hyper-torus Fusion Reactor, or from a Proto-Nitrate/BZ reaction. | 3.5 credits per mol. |
 Anti-Noblium |
Anti-Noblium is a rare gas used in high level Crystallizer recipes and as high tier fuel for the Hyper-torus Fusion Reactor. Outside of those uses, it doesn't do all that much. It does look pretty when in the air though! | Anti-Noblium can be made within the Hyper-torus Fusion Reactor when using Hyper-Noblium as the primary fuel with either Hydrogen or Tritium as the secondary fuel. Alternatively it can be made by electrolizing Hyper-Noblium with an electrolyzer machine. | 10 credits per mol. |
Physical Characteristics of Gases
TL;DR Gas flows from high pressure areas, to low pressure areas. Gas uses up more room when hot, less room when cold.
Ideal gas law: PV = nRT
Where R (ideal, or universal, gas constant) = 8.31, the following are linked by this equation.
Pressure (P): Measured in kPa, kiloPascals, Pressure is lethal above 750 kPa's. A pressure in a room above 1000 kPa's necessitates internals to breathe properly.
Volume (V): Another unseen variable, Volume is how much the area/canister/tank or piped tank has space inside it. This helps dictate how much gas it can hold. Volume is essentially the 'mole divider' when converting between a canister/air pump to your tank; having a higher volume essentially makes the tank that much more efficient, proportionally, so an Extended Emergency Oxygen Tank has twice the contained air per kPa in comparison to a regular Emergency Oxygen Tank.
Moles (n): Moles are the amount of particles of a gas in the air. It is moles that cause odd effects with a certain chemical. As it dumps so many moles to a tile, to keep the pressure acceptable, the moles have to be very, very cold, causing the infectious effect. Moles can be calculated by a form of the ideal gas law. n=(P*V)/(R*T)
Temperature (T): Measures in K, Kelvin, Temperature above 360 K and below 260 K causes burn damage to humans. Canisters rupture when the air surrounding them is over 1550 K.
Heat Capacity: A gasmix has heat capacity, and it is calculated by taking into account the quantity of all of the gases in the air and their specific heat. Heat capacity defines how much energy it takes to raise the temperature of a gas. The normal air mix (%30 O2, %70 N2) has a specific heat capacity of about 20 which doesn't impede heat transfer very much. Fires spreads quicker in gases with low heat capacity, and slower in gases with high heat capacity.
| Gas | Specific heat capacity (molar) |
|---|---|
| O2 | 20 |
| N2 | 20 |
| CO2 | 30 |
| N2O | 40 |
| Plasma | 200 |
| Tritium | 10 |
| Water Vapor | 40 |
| Hydrogen | 15 |
| BZ | 20 |
| Pluoxium | 80 |
| Miasma | 20 |
| Nitrium | 10 |
| Freon | 600 |
| Hypernoblium | 2000 |
| Proto-Nitrate | 30 |
| Halon | 175 |
| Healium | 10 |
| Zauker | 350 |
| Helium | 15 |
| Antinoblium | 1 |
Fire: An effect caused by the ignition of plasma, tritium, and hydrogen in an oxygenated room. It causes massive burn damage, and raises the temperature of the room.
In short the colder the gas and the higher the container volume, the more moles you can fit inside. This is why hot gases clog the red waste pipes - they expand, allowing fewer moles to be transported.
Optimizing Internals
- On a basic view, a 16 kPa minimum O2 requirement in internals. Pure O2 is theoretically toxic in real life, but has no representation for this in code, and takes a while to be really dangerous anyway (they use it to treat certain diseases, for example), and thus using a tank filled with air for internals is fairly inefficient.
- Cold O2 has more moles per kPa, and because people breathe in moles, and filling tanks usefully for internals are largely capped by the 1000 kPa release pressure, means cooling your O2 before using it in internals is important! Cooled down O2, such as from a freezer-ed canister, is the most efficient way to set up internals. Cooling it below 264 K will result in icicles inside in your lungs, though!
- If you need to empty an internal tank to make space for better, colder O2, you can use an Air Pump. Set it to "pump in" and "turn on" then "off" with the tank inside it, making it completely empty, thus allowing you to refill the tank more effectively.
- An emergency oxygen tank with normal settings lasts for about 12 minutes. Same tank, but with optimized gas temperature and output settings reduced, lasts about 50 minutes. If you don't have resources to get cooled O2 right now, set your output pressure to 16 kPa, it will give you 31 % more time to breathe.

Thermomachines
Combined entity of freezer and heaters, thermomachines allow you to influence the temperatures of gases connected to it. Thermomachines heat or cool the gas in their port to the target temperature.
An ideal thermomachine working at full efficiency "combines" the gas mixture actually present inside it with a gas mixture of a set temperature (depending on the user input) and heat capacity (depending on the quality of matter bins present). A heater will always work under the aforementioned ideal circumstances, while a cooler can get throttled down to a minimum of 65% efficiency.
Thermomachines need to have gas present inside it to properly cool or heat.
Crystallizer
The Crystallizer is a machine that allows gases to be solidified and made into various materials.
The working principle and gaseous requirements behind the crystallizer is rather simple and explained in the machine itself. You select a recipe, pump gases in using the input (green) port, meet the temperature requirements, and wait for the material to finish crafting. The red port is used for heat control, as it will conduct with the internal mix and influence the temperature. You have a 10% wiggle room for the temperature requirements, but straying too far from the optimal temperature will influence the final quality of the item produced. Quality affects the amount of gas consumed for each product produced, with higher qualities consuming less gas. The optimal temperature for any given recipe is the median between the lower and upper temperature bound. Stay as close as you can to the median value, and you'll be able to save up to 85% of the required gas if you manage to make the highest quality!
It currently supports the production of:
| Item | Properties |
|---|---|
| Metallic Hydrogen | A crafting material used for golems, axes, or the Elder Atmosian armor set. |
| Hyper-Noblium Crystal | A two use potion that can be used to pressure-proof two clothing items, turning them an icy blue color. |
| Healium Crystal | A grenade that can fix a large are of air to more reasonable temperatures. Good for firefighting and freezing temperatures. |
| Proto-Nitrate Crystal | A grenade that can refill a room with nitrogen and oxygen with a 8:3 split respectively. |
| Nitrous Oxide Crystal | A grenade that will release Nitrous Oxide (Laughing Gas). |
| Hot Ice | A material normally prodouced by freon combustion, also craftable with the Crystallizer.
When weldered or burnt, it rapidly releases a very hot and dense cloud of plasma gas. |
| Ammonia Crystal | A material with not much use except for exporting. Makes 2 every time it's completed. |
| Supermatter Shard | A smaller version of the extremely dangerous Supermatter Crystal used for power generation. |
| Diamond | A sheet of diamond. Many crafting and manufacturing applications. Used thorough the station. |
| Plasma Sheet | A sheet of plasma. Many crafting, manufacturing, and biological applications. Used thorough the station. |
| Crystal Cell | A non rechargeable cell with a huge power capacity. Rated at 50 MJ. |
| Zaukerite | A material without much use except for bragging rights. Makes 2 every time it's completed. |
| Drone Fuel Pellets | Three separate recipes that create Fuel cells used for drone explorations in Cargo.
Comes in three qualities denoting the difficulty of gases used. |
| Crystal Foam Grenade | A grenade that functions exactly like the standard Smart Metal Foam grenades that can be found roundstart.
Extremely simple to make compared to most other recipes. |
| Nitrium Crystal | A grenade that on detonation releases a chemical smoke cloud containing chemical Nitrium and Nitrosyl Plasmide. |
Bluespace Gas Vendor
This is the hub for gas purchase done by the crew. You only need to pipe your gas in and it will distribute it across vendors available station-wide. You can also set a price per mole for the gases. The gas will be at 20 degrees Celcius and is capped at 1013 kPa when purchased by the crew, do keep this in mind. You can also refill the individual vendors with metal for it to make more tanks.
After the Work is Done
This is a section dedicated to various tips and tricks, trivia, and things that you could do in your spare time:
- First and by far most important: make sure pipes don't get broken and if they do, fix them.
- Go around swiping your ID on Air Alarms, setting the operating mode to contaminated, and then re-swiping to lock it. You can ask the AI to do this as well, and probably should.
- Fill all the air pumps with air using a volume pump, these work until 9000 kPa compared to the pressure pump's 4500.
- Go to the red lockers, get a hard hat, gas mask and everything else that might be of use. Remember that you need both a fire suit and a hard hat to be resistant to weak fires. One will be useless without the other.
- Go grab the Fire Axe from the wall mount and hide it somewhere so the chucklefucks won't get it and go killing. DON'T take it with you and go walking through the hallways trying to look like a badass, you'll be the prime target of any antagonist/griffon who needs an efficient weapon.
- Least importantly, maintain the disposals system. You can generate pipes, but it needs welding and is generally a pain in the ass. You can also make fun slides, though.
- Using H/E pipes in space you can cool things down to a very low temperature very quickly. By making a cross with two off them you can have two on
- Gas pumps are for precise pressure control, volumetric pumps are for really fast pumping, and passive gates are for having 'one way' manual valves.
- Extremely high-temperature gases (like those from a panic siphoned fire) can really clog the waste loop. Could you do something to correct that?
- No one uses the ports outside of the 'refilling' station, but that doesn't mean that functionality can't be added onto them!
- The wall section that looks like the letter 'I' can be dismantled if you need more working space for pipes.
- Don't count out the grated window areas, they can be a great (har har) way to utilize the vacuum of space without an EVA suit.
- Speaking of EVA suits, your engineering buddies can potentially help you with anything you might want to do in space, be it adding or modifying pipes. Watch the hilarity as that incompetent engineer fumbles when setting up a heat exchanger pipe loop!
- The mining station doesn't have air recycling. Very long rounds might make this a problem for any miners working there.
- Any gas at pressure over 1000 kPa will cause you to start suffocating as in a vacuum. You can just use internals, though.
- N2O is invisible at low pressures. If you start giggling, put on your internals to avoid passing out.
- Any gas can displace O2, and less than 16 (also useful for optimizing internals) kPa of oxygen starts the Oxyloss. CO2 can be removed with the scrubbers, but to get rid of N2 simply apply some way of removing gas from the air and adding O2. My personal favorite is 2 air pumps, 3 connectors and an Air Filter and a canister: 1 pump draws in, goes through the connection and filters N2 into the canister, and the rest to the other pump, which expels it. Can also be used for N2O which is only sluggishly scrubbed otherwise.
- Pressures above 750 kPa do 10 DPS + 5 DPS for every extra 375 kPa above that mark, rounded off. Space suits completely block it all, but there is no other defense.
Atmospherics 301: Pipenet Theory and more
LINDA? What is LINDA? LINDA is our atmospherics system. There are various theories on the origin of this name, but that is not why we are here. We are here to understand how gas dissipate, how pipelines and eventually pipenets are formed, and the more technical parts of atmospherics.
For a technical and detailed breakdown of our atmospherics subsystem and how everything works, refer to https://github.com/tgstation/tgstation/blob/master/code/modules/atmospherics/Atmospherics.md
This will be an abridged version of that guide, intended for those without any experience in codediving. This not a substitute for that guide however, and we highly implore you to visit the documentation, even if you don't have any experience in reading code-heavy documentations.
We will use the supermatter and various figures as scenarios, this guide is best read once you know the basics of how supermatter works and have read the guide.
Pipeline and Pipenet Theory
If there is one part of Atmospherics 301 that you should read, this is it.

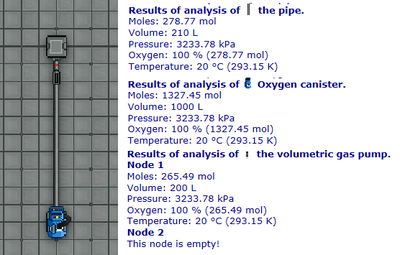
Our atmospherics susbystem does not simulate flow. Every interlinked pipe is connected into a single pipeline, and every pipeline member is subject to gas sharing. That is to say that when you use your Analyzer on a single pipe, what you are seeing is the gas mixture of the pipeline (this returned gas mixture information doesn't include gas inside of machineries like pumps, only the regular pipes, but they are considered as extra member of the pipeline and are subject to the same gas sharing rules.)
(Extra note: in the code, we store gas information for each calculated atmospheric entity in datums called gas mixtures, this is important terminology right here, but you don't need to worry too much about this.)
Each pipeline have a gas mixture, each individual member of the pipeline gets a share of this big gas mixture based on how much their share of the total volume is. Lets take the picture on figure 3.2 as an example. Notice that the pressure is preserved on all the analyzed machineries, demonstrating that once again: the gas is being shared equally between all of them based on their respective volume. This is what the supermatter guide refers to as gas directly appearing in the two ends of the pipeline as wide as the observable universe. Keep this concept of gas sharing in mind!
Most atmospheric devices perform actions only the gas directly under their influence; the gas that are directly present in their system. You can take a look back at the volume pump in figure 3.2, the volume pump will only move the gas directly inside their gas mixture. This is why taking gas out of the huge distribution loop is tedious and long, this is why some supermatter setups will lack moles directly inside the chamber if you expand the cooling space loop too much, this is why thermomachines are less reliable on very huge pipe networks.
Layer manifolds and valves connect different pipelines together by doing gas redistribution. Pumps however, does not. They have two gas mixtures that when connected, will be part of two separate pipelines. These pipelines will not interact with one another, but the gas will be allowed to move in one direction when the pump is turned on. You can imagine these with cups of water and salt being mixed in various forms and being put sideways; valves and manifolds combine both the cups by gluing it, while pumps work by adding a filter paper and only allowing water to flow into the salt cup and not in the reverse direction. This is why the supermatter guide goes into length on removing pumps, because they are only there to restrict flow and create pressure gradients, not to mention how they are prone to clogging.
We go into great lengths in explaining pipelines and pipenets because it is very important and will show up again and again in your journey as an atmospheric technician. Now buckle up for a shorter exposition of environmental atmospherics!
LINDA: Active Turfs and Excited Groups
Our atmospherics system: LINDA, work based of concepts of active turfs and excited groups. A turf (tile) will become active when any gas changes happen, be it a plasma canister being opened, a breach occuring, or as simple as scrubber taking CO2 out. A turf will also become activated if a wall is deconstructed, necessitating it to be filled. These active turfs will combine into an excited group and equalize every several iteration of the subsystem ticking. This is LINDA in it's simplest, most abridged form.
Superconduction
On high temperatures (above 100 degrees Celcius), superconduction occurs. Superconduction allows heat to transfer between closed turfs, it is what allows glass to break on plasma fires, and why windows are really bad insulators. In short, superconduction is the interface between the turf and the gas mixtures. You will not need to understand the inner workings of this phenomenon during your course as a technician, but keep in mind of it's effects.
There is however, one exception: super hot turf based fires (incinerator springs to mind) As previously stated, superconduction is the interface between the turf level and the gas mixtures, the turfs are initialized at 20 degrees celcius and with a heat capacity of, well, practically infinite. This means that on every single instance of your gas mixture being hotter than 100 degrees celcius, it is sharing with the 20 degree celcius turf (losing heat, or why canisters fires are hotter than incinerator). This presents an extra challange for you, our dearest technician, in making very hot fires.